 Home
Home
 Back
Back
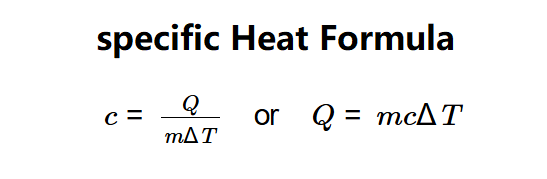
This tool determines either the specific heat capacity (\( c \)) of a substance or the thermal energy (\( Q \)) needed to alter its temperature, utilizing the equation \( c = \frac{Q}{m \Delta T} \) or \( Q = m c \Delta T \).
It plays a key role in fields like physics, engineering, and thermodynamics for evaluating material thermal characteristics or energy demands in temperature adjustments, supporting thermal system designs and substance evaluations.
The tool relies on the specific heat equation:
Key variables include:
Process Overview:
Performing calculations for specific heat or thermal energy is essential for:
Sample 1 (Thermal Energy for Argon): Determine the energy to modify Argon's temperature:
Sample 2 (Specific Heat Determination): Find the specific heat capacity for a substance:
Q: What does specific heat capacity mean?
A: Specific heat capacity (\( c \)) refers to the heat needed to increase the temperature of 1 kg of a substance by 1 Kelvin (or 1°C), indicating how well it retains thermal energy.
Q: What is the role of temperature change?
A: Temperature change (\( \Delta T \)) represents the shift (\( T_f - T_i \)), influencing the quantity of heat gained or lost by the substance.
Q: Why support calculations for both \( c \) and \( Q \)?
A: The equation \( Q = m c \Delta T \) allows rearrangement to isolate either \( c \) or \( Q \), offering flexibility for various needs in the tool.